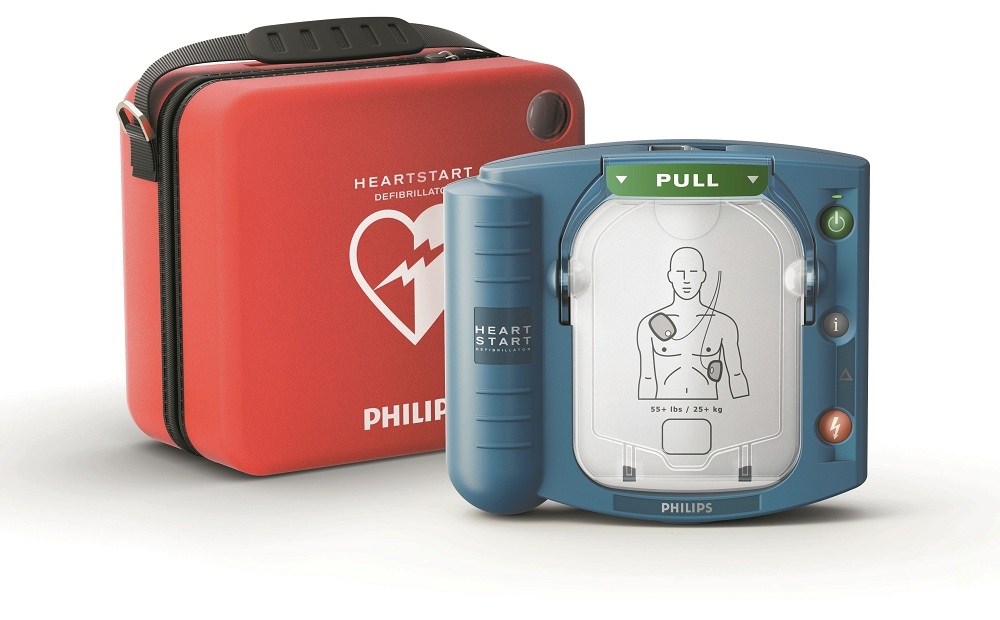
Defibrillatore per Primo Soccorso HeartStart HS1
Vi dà la sicurezza nel sapere che potete salvare una vita umana anche prima che arrivino i soccorsi.
Concepito per l'utilizzo da parte di chiunque, ovunque e in qualsiasi momento.
Informazioni Prodotto
Vi dà la sicurezza nel sapere che potete salvare una vita umana anche prima che arrivino i soccorsi.
Concepito per l'utilizzo da parte di chiunque, ovunque e in qualsiasi momento.
Facile da usare
Istruzioni impartite con voce naturale, in tono calmante, dirigono il primo soccorritore nelle operazioni di defibrillazione e RCP di pronto soccorso.

Istruzione a voce
Aiuta l'utilizzatore a prendere il controllo della situazione mediante istruzioni semplici da seguire impartite con voce chiara e sicura.
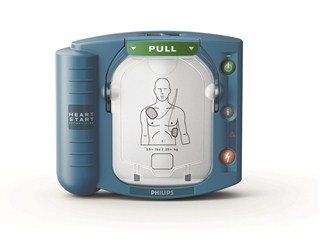
Facile da usare
Istruzioni impartite con voce naturale, in tono calmante, dirigono il primo soccorritore nelle operazioni di defibrillazione e RCP di pronto soccorso.

Sempre pronto per l'uso
Grazie a degli auto-test completi, tutte le funzioni vitali dell'HeartStart vengono automaticamente verificate secondo un calendario giornaliero, settimanale e mensile, in modo da garantire che l'HeartStart sia sempre pronto per il soccorso d'urgenza.

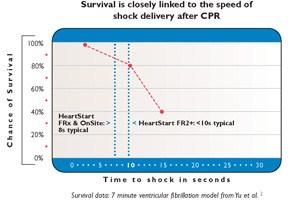
Quick Shock feature
Minimize chest compression interruptions
CPR is even more vital to survival than previously realized. Rapidly delivering a shock after chest compressions is critical. The HeartStart HS1’s Quick Shock feature reduces the time between hands-off and shock delivery.
Integrated SMART pads
Senses application to patient skin The pads are "smart" because they can sense that they have been removed from the cartridge and applied to the patient. This helps the HeartStart OnSite know at what point the user is in the response process.
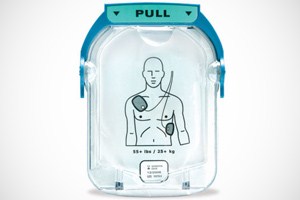
Visual queues
Clear, intuitive icon driven operation
The HeartStart HS1’s graphics-based visual instructions are designed to communicate instantly, with minimum reading and interpretation. Users not fluent in the device’s language can follow easily.

Specifiche Tecniche
Product Specifications
Defibrillator Number
Defibrillator Family
- HS1
How Supplied
- Defibrillator, battery (one, preinstalled), SMART Pads (one, preinstalled), Set-up/Maintenance guide with expiration date tags, Owner’s Manual, Quick Reference Guide.
- HeartStart Onsite (HS1) Ready-Pack option R01: Defibrillator inside carry case, battery (one, pre-installed), SMART Pads (one set pre-installed plus spare set in carry case), Set-up/Maintenance guide with expiration date tags, Owner’s Manual, Quick Reference Guide.
Waveform
- Truncated Exponential Biphasic. Waveform parameters adjusted as a function of each patient’s impedance.
Therapy
- Adult defibrillation peak current: 32A (150J nominal) into a 50 ohm load. Pediatric defibrillation (with optional Infant/Child SMART Pads Cartridge): 19A (50J nominal) into a 50 ohm load.
Shock-to-Shock Cycle Time
- Typically less than 20 seconds between shocks in a series.
Quick Shock
- Able to deliver a shock after the end of a CPR interval, typically in eight seconds.
Voice Instructions
- Detailed voice messages guide responder through use of the defibrillator.
CPR Coaching
- Instructions for adult and infant/child CPR available at user’s option. Shock Delivery Via adhesive pads placed on patient’s bare skin as illustrated on pads.
Controls
- Green SMART Pads cartridge handle, green On/Off button, blue i-button, orange Shock button.
Indicators
- Ready light; blue i-button; caution light.
Physical Requirements
Sealing
- Solid objects per EN60529 class IP2X Drip-proof per EN60529 class IPX1
Temperature
- Operating: 32º - 122º F (0º - 50º C)
- Standby: 50º - 109º F (10º - 43º C)
Humidity
- Operating: 0% to 95% relative, non-condensing
- Standby: 0% to 75% relative, non-condensing
Altitude
- Operating: 0 to 15,000 ft (0 to 4,500 m)
- Standby: 0 to 8,500 ft > 48 hours and 8,500 to 15,000 feet < 48 hours
Shock/Drop
- Abuse Withstands 1 meter drop to any edge, corner or surface.
Vibration
- Meets EN1789 random and swept sine, road ambulance specification in operating and standby states.
EMI (Radiated/Immunity)
- Meets EN55011 Group 1 Level B Class B and EN61000-4-3.
Patient Analysis System
Patient Analysis
- Evaluates patient ECG to determine if a rhythm is shockable. Rhythms considered shockable are ventricular fibrillation (VF) and certain ventricular tachycardias (VT) associated with lack of circulation. For safety reasons, some VT rhythms associated with circulation will not be interpreted as shockable, and some very low-amplitude or low-frequency rhythms will not be interpreted as shockable VF.
Sensitivity/Specificity
- Meets AAMI DF80 guidelines and AHA recommendations for adult defibrillation (Circulation 1997;95:1677-1682).
Artifact Detection
- The effects of pacemaker artifact and electrical noise are minimized with artifact detection.
Battery Type(M5070A)
- 9 Volt DC, 4.2 Ah, composed of disposable long-life lithium manganese dioxide primary cells. Capacity Minimum 200 shocks or 4 hours of operating time (EN 60601-2-4:2003)
Install-By-Date
- Battery is labeled with an install-by date of at least five years from date of manufacture.
Standby Life
- Four years typical when battery is installed by the install-by date. (Will power the AED in standby state within the specified standby Temperature range, assuming one battery insertion test and no defibrillation uses.)
SMART Pads
Adult SMART Pads Cartridge
- M5071A defibrillation pads for patients 8 years of age and older or 55 lbs. (25 kg) and over.
Infant/Child SMART Pads Cartridge
- M5072A defibrillation pads for patients under 8 years of age or 55 lbs. (25 kg). Rx only.
Energy Delivered
- Adult: nominal 150 Joules into a 50 ohm load
- Infant/Child: nominal 50 Joules into a 50 ohm load
How Supplied
- Disposable cartridge, containing adhesive defibrillation pads, clicks into defibrillator for an integrated pads solution.
Active Surface Area
- 13.2 in² (85 cm²) each
Cable Length
- Adult pads: 54 in (137.1 cm)
- Infant/Child pads: 40 in (101.6 cm)
Use-by Date
- Cartridge is labeled with a use-by date of at least two years from date of manufacture.
Training Pads
Adult Training Pads Cartridge
- M5073A
Infant/Child Training Pads Cartridge
- M5074A
Function
- Special pads put HeartStart OnSite into training mode and disable its energy delivery capability. Training pads feature 8 real-world training scripts. Used with training mat (included) or with adapters on manikins.
Automated and User Activated Self-Tests
Daily Automatic Self-Tests
- Tests internal circuitry, waveform delivery system, pads cartridge and battery capacity.
Pads Integrity Test
- Specifically tests readiness-for-use of pads (gel moisture).
Battery Insertion Test
- Upon battery insertion, extensive automatic self-tests and user-interactive test check device readiness. Status Indicator Blinking green "Ready" light indicates ready for use. Audible “chirp” indicates need for maintenance.
Data Recording and Transmission
Infared
- Wireless transmission of event data to a PC or Palm® PDA, using the IrDA protocol.
Data Stored
- First 15 minutes of ECG and the entire incident’s events and analysis decisions.